Why is gamma decay more dangerous than alpha decay or beta decay?
1 Answer
That is actually not necessarily true!
Explanation:
Alpha-, beta- and gamma-radiation have different penetrating ability, this is often linked to 'risk' or 'danger', but that is often not true.
First let us take a look at the penetrating ability of the different types of radiation:
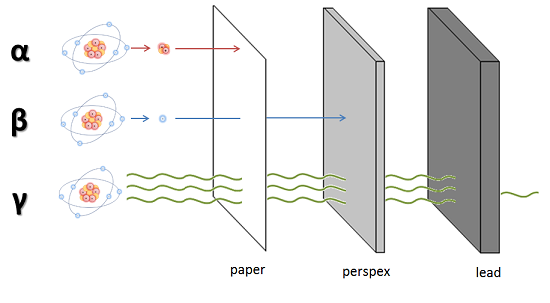
- Alpha (
#alpha# ): large particles (2 neutrons, 2 protons); +2 charge - Beta (
#beta# ): smaller (electron); -1 charge - Gamma (
#gamma# ) or X-ray: a wave (photon); no mass, no charge
Because of their mass and charge alpha particles are easily stopped by a piece of paper and even the top layer of your skin. The smaller beta particles can travel a bit further and can be stopped with a layer of perspex.
For gamma rays it is a very different situation, because it is a wave (such as light and sound) and has no mass and charge. In theory a wave can travel forever in material. Interaction with material is a chance process. Usually a layer of lead or a thick layer of concrete is used to reduce transmission to a reasonable level.
Only looking at the penetrating ability, gamma rays might seem more dangerous because they can travel much further. This isn't always the case:
That alpha particles are easily stopped doesn't meant they have less energy. It only means they lose their energy on a very short distance. When you ingest or inhale these particles they can cause a lot of damage.
A beta particle can also do a lot of damage when they are inside your body and also on the skin and for example the eyes (risk of cataract).
A high energy gamma ray can easily enter your body, but it can also just as easy exit your body. It usually causes less damage on its way!
So it is not the radiation itself that makes it 'dangerous', its just that alpha and beta particles are easier to shield then gamma rays.

