A 4.08 g sample of a compound of nitrogen and oxygen contains 3.02 g of oxygen. What is the empirical formula?
1 Answer
The empirical formula is
Explanation:
An empirical formula is the lowest whole number ratio of elements in a compound. Each ratio will be the subscript of each element.
Determine the mass of each element in grams.
Determine moles of each element by dividing the mass of each element by its molar mass (atomic weight on the periodic table in g/mol.
Determine mole ratios by dividing the moles of each element by the least number of moles.
The empirical formula is
The compound with this formula is called nitrosylazide with the following structure.
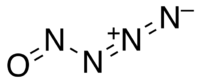
For more examples of this type of empirical formula problem, check out the following website: http://www.chemteam.info/Mole/Emp-formula-given-mass-data.html
For examples of determining an empirical formula from percent composition, check out this website: https://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm

