What is the formula of ammonium carbonate?
1 Answer
Explanation:
The important thing to recognize here is the fact that you're dealing with two polyatomic ions, one which acts as cation and one which acts as anion.
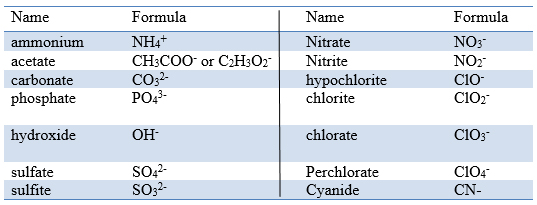
The name of the cation is always added first to the name of the ionic compound. Likewise, the cation is added first to the compound's chemical formula. In this case, you know that you have ammonium,
The name of the anion follows the name of the cation. In this case, you know that you have the carbonate ion,
Now, notice that the anion carries a
In this case, you need two ammonium cations to balance the
#2 xx ["NH"_4^(+)]" "# and#" "1 xx ["CO"_3^(2-)]#
which means that the chemical formula for this compound will be
#("NH"_4)_2"CO"_3 -># ammonium carbonate

