How many molecules of water are there in 36 grams of H_2O?
1 Answer
Explanation:
To go from the mass of water to molecules of water, we have to do two things:
-
Convert mass of
H_2O to moles ofH_2O using the molar mass ofH_2O as a conversion factor -
Convert moles of
H_2O to molecules ofH_2O using Avogadro's number (6.02xx10^(23) ) as a conversion factor
Before we start, I should note that the molar mass of
We can go from mass to moles using dimensional analysis. The key to dimensional analysis is understanding that the units that you don't need any more cancel out, leaving the units that are desired:
We'll use the following relationship:
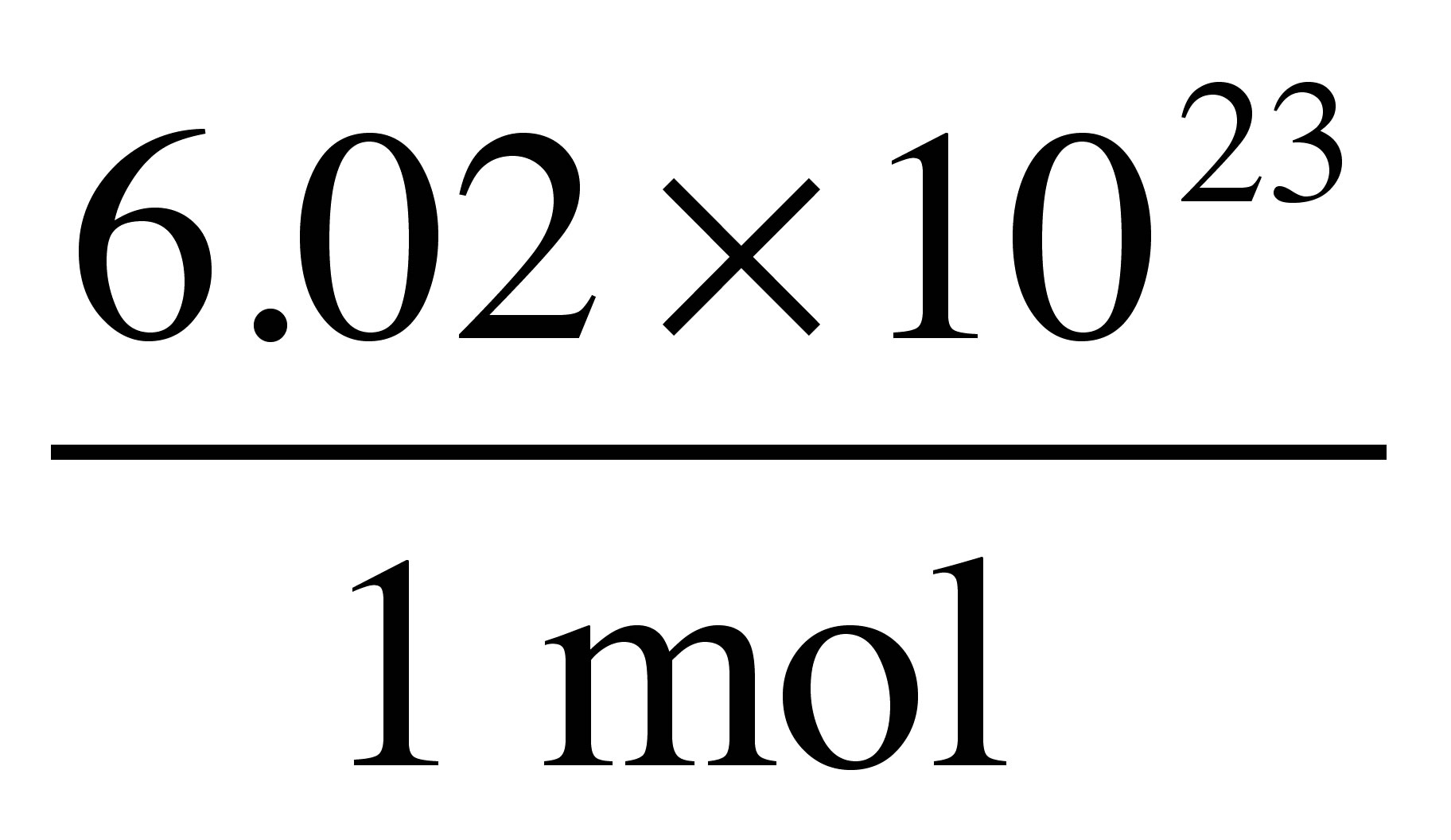 www.sprinklernewz.us
www.sprinklernewz.us
Using the moles of

