The mass number of a chromium atom is 52 and it has 24 protons. How many neutrons does this atom have?
1 Answer
Aug 7, 2016
Chromium has 28 neutrons
Explanation:
To answer this question, we have to use the formula below:
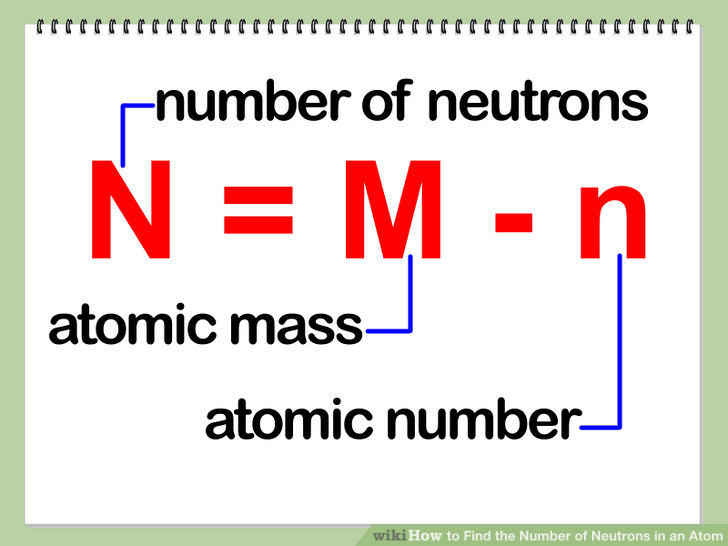 www.wikihow.com
www.wikihow.com
We know the mass number and the atomic number. The mass number in our case is 52 and the atomic number, which is equal to the number of protons in the atom's nucleus, is 24.
All we have to do is subtract 24 from 52 to obtain the number of neutrons like this:
Thus, the atom contains 28 neutrons

