The structural formula is how the molecule is actually drawn out, but this is a fairly simple molecule.
In the case of #"PH"_3#, you know that hydrogen only has one electron, and so it can only make a maximum of one single bond (most of the time).
Therefore, phosphorus (#"P"#) must be the central atom, and you just draw hydrogen atoms around it. Phosphorus is right below nitrogen, so it has the same number of valence electrons as nitrogen.
As a result, #"PH"_3# (phosphorus trihydride) and #"NH"_3# (ammonia) are isoelectronic (same number of electrons, or similar electronic structure), and they have the same molecular geometry as well:

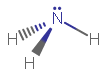
(bond angles approximately to scale.)


