With a 0.5 M solution, many moles of NaCI would there be in 1,000 mL?
1 Answer
Aug 18, 2016
Explanation:
Let's begin with the equation for molarity:
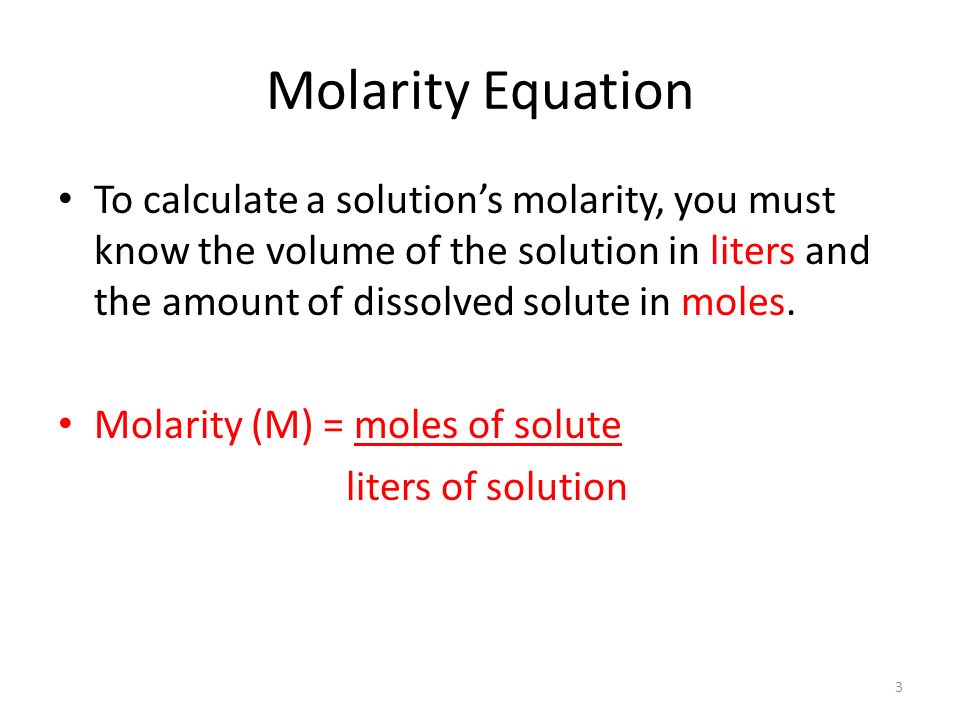 slideplayer.com
slideplayer.com
We are given the molarity and the volume of solution. The only issue is that the volume is given in mL instead of L. This issue can be fixed by using the following conversion factor:
Therefore, if we divide 1000mL by 1000mL we will obtain a value of 1L.
Rearrange the equation to solve for moles of solute:
Moles of solute = Molarity
Multiply 0.5 M by 1 L:
Boom, there would be 0.5 moles of NaCl!

