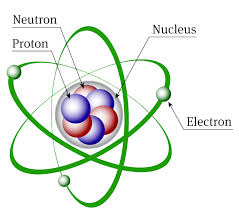
What is a particle with zero charge found in the nucleus of an atom is called?
1 Answer
Sep 7, 2016
It is called a neutron because it is neutral.
Explanation:
The nucleus of an atom contains protons and neutrons. The protons are positively charged and the neutrons have no charge. The electrons are located outside of the nucleus in the electron cloud.