Question #59964
1 Answer
Sep 24, 2016
2
Explanation:
The Lewis dot structure of Methoxymethane
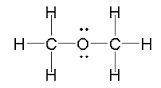
The Oxygen atom has two unpaired and two lone pairs of electrons in the valence shell.The two unpaired electrons form two

