How does boiling point depend on pressure and temperature?
1 Answer
If you are asking for the definition of a boiling point then we can help.
Explanation:
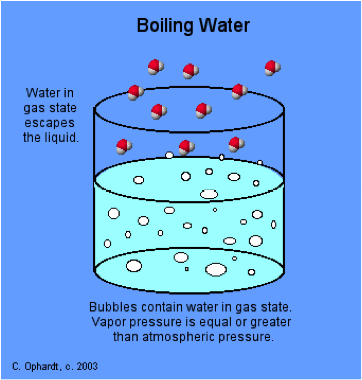
The boiling point of a liquid is the temperature at which the vapour pressure of the liquid is equal to the ambient pressure, and bubbles of vapour (= gas) form directly in the liquid.
The