Why do we multiply molar concentrations and not sum them, and why there's no squared sign here?
From Wikipedia's page Antigen-Antibody Interaction
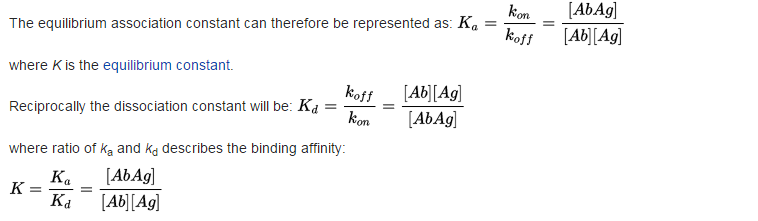
Why do we multiply [Ab] and [Ag] and not, say, sum them up?
Why the final term has no squared signs? We've basically multiplied two similar terms.
From Wikipedia's page Antigen-Antibody Interaction

Why do we multiply [Ab] and [Ag] and not, say, sum them up?
Why the final term has no squared signs? We've basically multiplied two similar terms.
1 Answer
By the law of mass action rate of reaction in certain direction is proportional to the active mass or the molar concentration of the reactants.So following the law of joint variation the molar concentrations (of reactants) ralsed to proper powers are multiplied to get the rate of reaction in certain direction.
Here the reacion is represented as follows
where
Again

