What do all atoms of all elements have in common?
1 Answer
Oct 17, 2016
The common feature is that the atoms of all elements consist of electrons, protons, and neutrons.
Explanation:
All atoms have a dense central core called the nucleus.
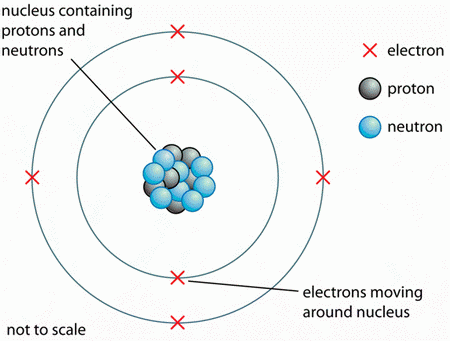
(From getrevising.co.uk)
The nucleus consists of two kinds of particles:
- protons, which have a charge of +1
- neutrons, which have no charge.
Surrounding the nuclei of all atoms are the electrons, which have a charge of -1.

