Question #a6cdc
1 Answer
The enthalpy of formation of liquid benzene are obtained by applying the law of Hess. The resulting value is:
Explanation:
Enthalpy is a state function. This means that their variation depends only on the initial and final states but not of the path to go from one to another. Hess's law says just that, we can calculate the enthalpy change of a reaction using different paths.
For example, if we have to calculate the enthalpy change that undergoes a system when a chemical reaction,
takes place, we could choose an alternative path to obtain each substance from its pure elements (ie, we would use formation reactions of each substance), as shown in the following figure:
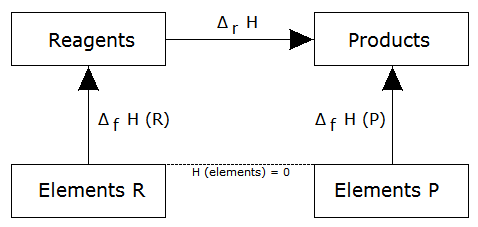
Then, the enthalpy of the reaction is equal to the enthalpy of formation of the products minus the enthalpy of formation of the reactants, ie:
formula which is often used as an immediate consequence of the law of Hess.
How we can apply this to the problem above? First, we must realize that the reactions of combustion of carbon and hydrogen are actually forming reactions of carbon dioxide and water:
And when we write the reaction of combustion of liquid benzene:
we can calculate the enthalpy change of this reaction as the difference between the sum of the enthalpies of formation of the products minus the sum of the enthalpies of formation of the reactants:
Substituting the known values of each enthalpy, we can calculate the enthalpy of formation of liquid benzene:
[Although the statement does not indicate, the enthalpy given above are actually per mole of substance, ie they are molar enthalpies of combustion. Therefore, the resulting units are

