Question #3a9fc
1 Answer
Nov 6, 2016
Explanation:
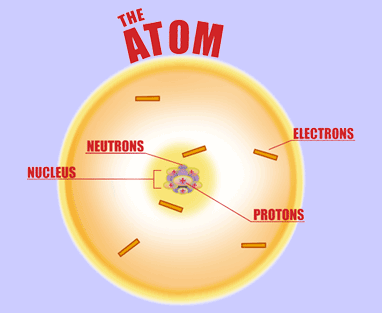
For starters, the protons are not orbiting the nucleus; they are, in fact, located inside the nucleus. The same can be said of the neutrons.
On the other hand, the electrons are surrounding the nucleus, i.e. they are located outside of the nucleus.
Now, if the atom is neutral, which is probably the case here, then you must always keep in mind the fact that
#color(blue)(bar(ul(|color(white)(a/a)color(black)("no. of protons " = " no. of electrons")color(white)(a/a)|)))#
That is always true for a neutral atom. In your case, you know that there are