What type of compounds cannot be detected using infrared spectroscopy and why?
1 Answer
Nov 8, 2016
In general, compounds that are symmetrical AND cannot form a polar shape (namely, all homonuclear diatomics), like
Note that
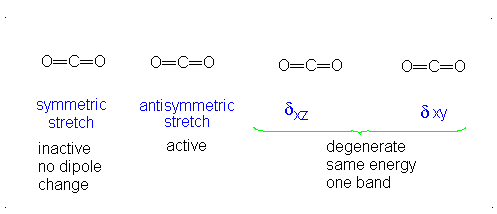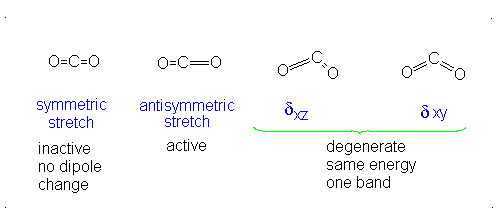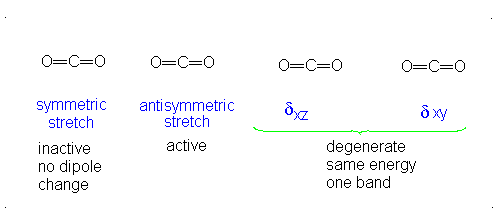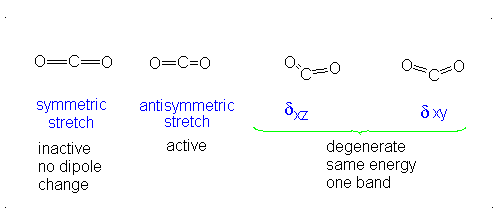
This change in dipole moment is detected by IR spectrometers, and so, peaks will show up (specifically near
Note that the first vibrational motion has no dipole change, so it is IR-inactive. The remaining three are IR-active.

