Question #ff017
1 Answer
Nov 11, 2016
Here's what I get.
Explanation:
Your text may have slightly different values for the enthalpies of formation.
For
For
Here's the enthalpy diagram I created in Excel.
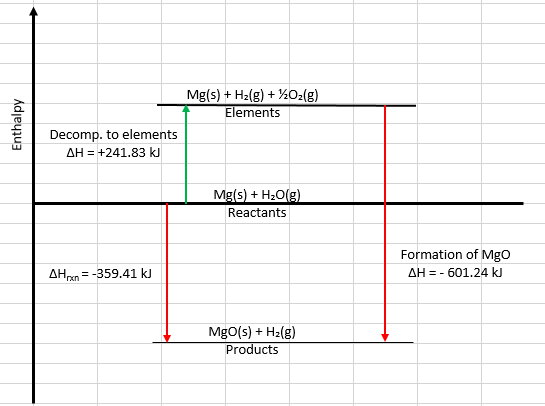
Step 1 is the decomposition of water into its elements (the green arrow).
Step 2 is the formation of MgO(s) from its elements (the red arrow).

