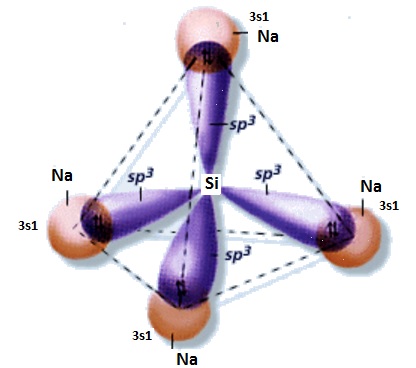
What is the Lewis dot structure of #Na_4Si#?
1 Answer
Nov 20, 2016
look on the periodic table of the elements Na and Si!
Explanation:
Silicon is in the same carbon family, so remember the organic chemistry of carbon, it has sp3 hybridization and makes 4 bonds.
The Lewis dot structure of
With sigma bonds (