Does dry ice melt or evaporate?
1 Answer
At room temperature and atmospheric pressure, neither one! It skips past the liquid phase at room temperature.
Here's what the phase diagram of
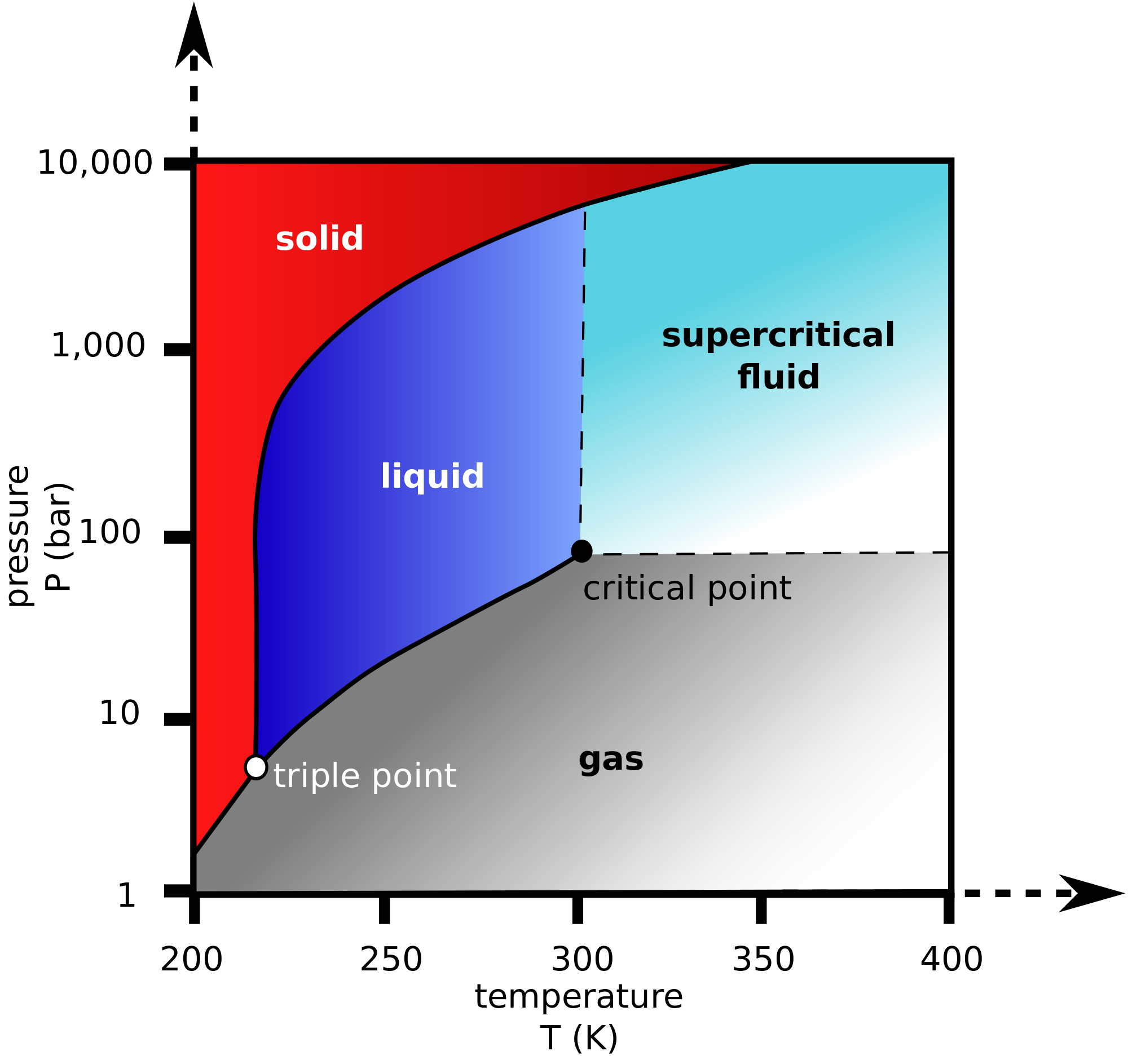
At
When exposed to room-temperature conditions, it thus sublimates, i.e. it spontaneously crosses the solid-gas equilibrium line on the above phase diagram and becomes a gas.
MELTING?
If we were to want dry ice to melt at room temperature, we would have to first increase the pressure to at least
But if one raises the temperature just a few more degrees, one would obtain a supercritical fluid, which means the liquid and gas phases are no longer distinguishable.
That is, initially one would see bubbles in a liquid, but eventually, the bubbles would disappear as the fluid becomes uniform.
EVAPORATING?
if we were to want dry ice to evaporate at room temperature, we would have to decrease the pressure slightly from
So, under normal conditions (i.e.

