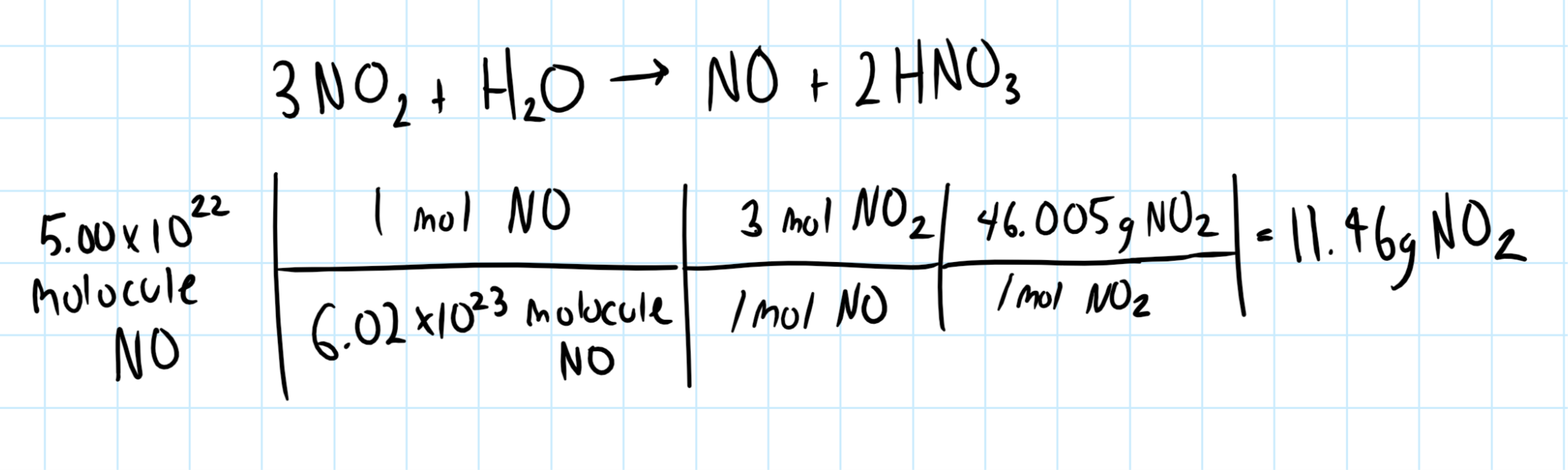
How many grams of nitrogen dioxide must react with water to produce #5.00*10^22# molecules of nitrogen monoxide?
1 Answer
Dec 6, 2016
11.46 grams
Explanation:
Consider the balanced chemical equation, which we need to obtain the molar ratios:
I see you would like to know the grams
Let's use the molar ratios to calculate the amount of nitrogen dioxide required.
Consider the graphic below: