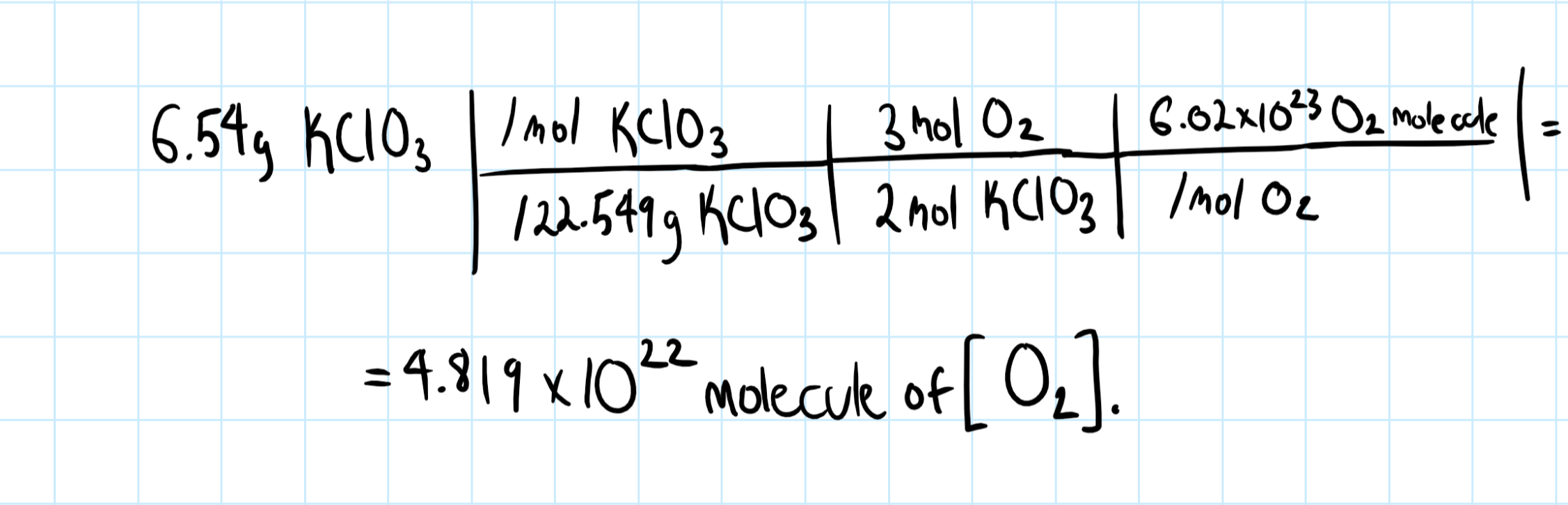How many molecules of oxygen are produced by the decomposition of 6.54 g of potassium chlorate in the reaction #2KClO_3(s) -> 2KCl(s) + 3O_2(g)#?
1 Answer
Dec 6, 2016
Explanation:
We have:
as a balanced chemical reaction.
I see you want to know the amount
Consider the graphic below: