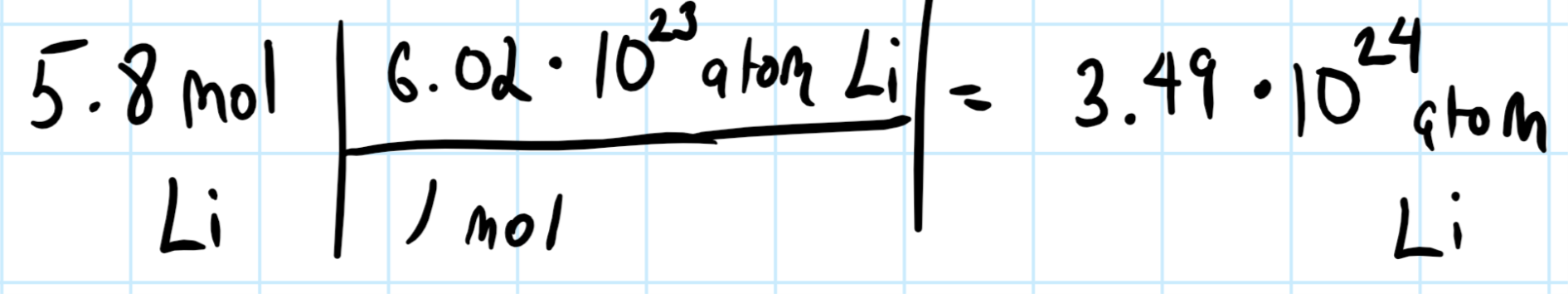
How many Li atoms are in 5.8 moles of Li?
2 Answers
Dec 9, 2016
Explanation:
And
What is the mass of this quantity of lithium atoms? Hint, 1 mole has a mass of
Dec 9, 2016
Explanation:
Consider: