A sample of glass that has a mass of 6.0 g gives off 12 J of heat. If the temperature of the sample changes by 4.0°C during this change, what is the specific heat of the glass?
1 Answer
Dec 18, 2016
Explanation:
To obtain the specific heat of that glass, let's use the equation below:
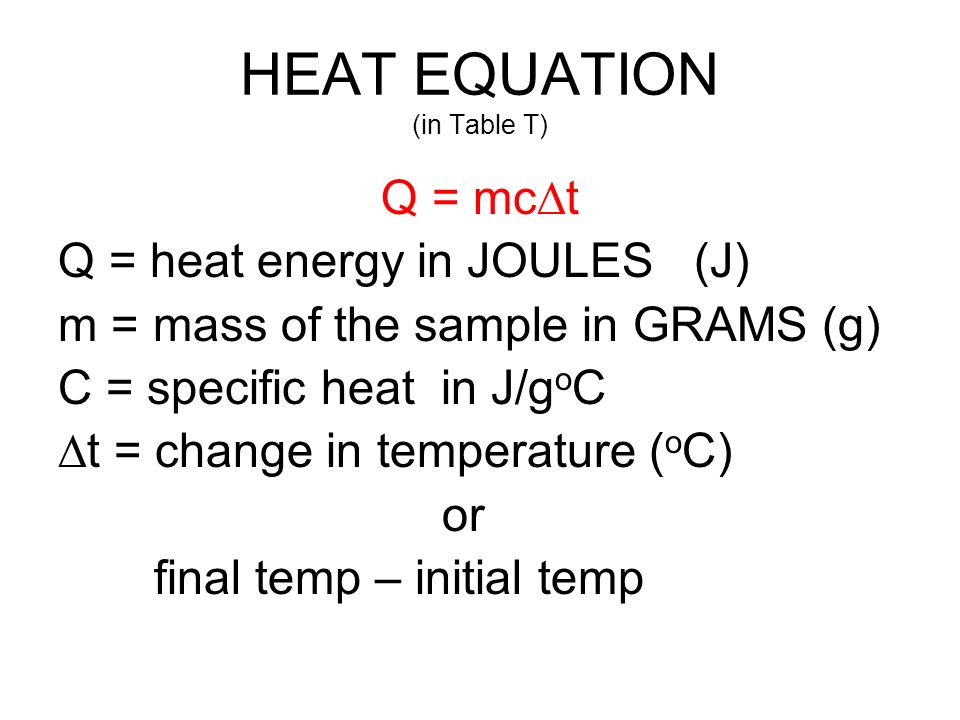
Based on the information you've provided, we know the following variables:
All we have to do is rearrange the equation to solve for
Now, we just plug in the known values:

