How many molecules are in 2.00 moles of #NO_2#?
1 Answer
Dec 29, 2016
Explanation:
We are going to use dimensional analysis (method that allows you to convert from one unit of measurement to another by cancelling out unwanted units) and the relationship below:
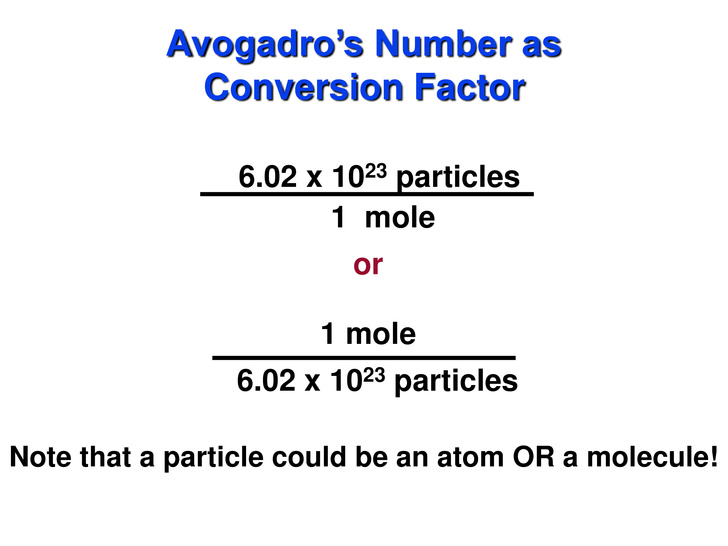
For questions like this I use the following technique:
Let's plug in our values:

