What is the mass percent of hydrogen for #C_3H_5OH#?
1 Answer
Explanation:
In order to compute the mass percent of hydrogen in
The formula mass of
The atomic mass of the hydrogen atom is
Now, we have to use the following equation:
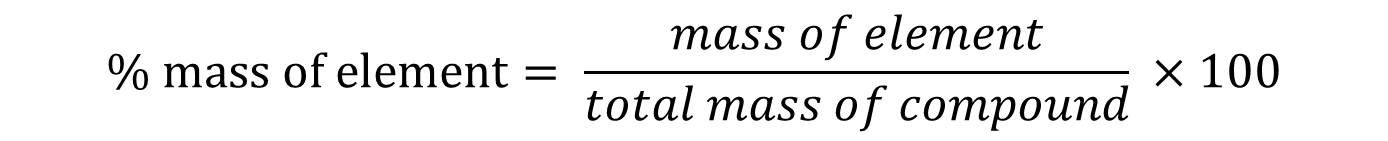
The numerator represents the mass of the desired atom, which is H in our case, and the denominator represents the mass of the entire compound. You just divide the two and multiply by 100 to obtain the percent composition:
I multiplied the atomic mass of hydrogen by 6 because you have to account for all of the hydrogen atoms in the formula.


