Which of these molecules is polar? #"SiF"_4#, #"XeF"_4#, #"BF"_3#, #"SF"_4#
1 Answer
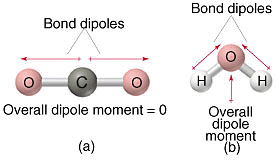
A polar molecule has both an uneven distribution of electrons and no total symmetry to counteract it. This image sums it up:
When a molecule has a great deal of symmetry, you can rotate it in certain ways that return it to an indistinguishable position, meaning that any polarity it has will cancel out.
(By an indistinguishable position, I mean that you can turn your back, someone else can rotate the molecule in a particular way, and when you turn around, you can't tell anything happened.)
Assuming you aren't very familiar with vectors...
For your purposes, if the molecule has any lone pairs of electrons, you can consider it not totally symmetric. Therefore, it will be polar by virtue of its shape, even if its individual bonds are not.
Using the valence electron-counting method, draw each of these Lewis structures.
#1)# #"SiF"_4#
Each
Therefore, we have four electron groups (of those four, all are bonding groups), giving a tetrahedral structure:
![]()
This is totally symmetric, so this is a nonpolar molecule, even without checking the polarity of each individual bond.
(If we did,
#2)# #"XeF"_4#
Each
Therefore, we have six electron groups, two of which are lone pairs, giving a square planar structure (take two atoms off of the same axis in an octahedral molecular geometry to derive it):
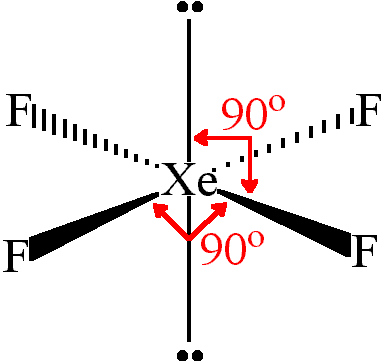
This is totally symmetric, because each
So, this is a nonpolar molecule, even without checking the polarity of each individual bond.
#3)# #"BF"_3#
I'll let you follow the previous parts of the answer to derive the molecular geometry, which is trigonal planar (recall that boron is OK with six valence electrons).
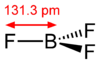
This is also totally symmetric, so this is nonpolar.
#4)# #"SF"_4#
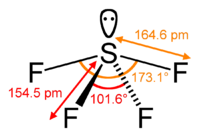
The
This is not totally symmetric, so this is a polar molecule.
In summary, for your purposes, if the molecule has any lone pairs of electrons, you can consider it not totally symmetric. Therefore, it will be polar by virtue of its shape, even if its individual bonds are not.