What is the formula for manganese iV bicarbonate?
1 Answer
Explanation:
Manganese (IV) bicarbonate is an ionic compound, composed of two ions, Manganese (IV), and bicarbonate, also called hydrogen carbonate.
Ionic compounds are neutral, meaning that the total positive charge and total negative charge are equal.
The name manganese (IV) tells us that the manganese ion has a charge of
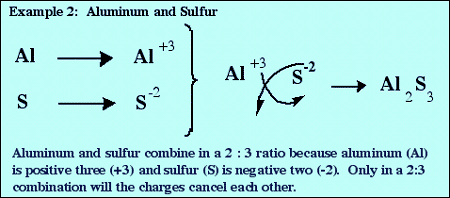
Another way to determine the formula of an ionic compound is by crisscrossing. The size of the charge on the positive ion becomes the subscript on the negative ion, and the size of the charge on the negative ion becomes the subscript of the positive ion.
The examples below show how the process works.