Classification of crystalline solids
(Criteria - Type of constituent particles and nature of bondage existing between them )
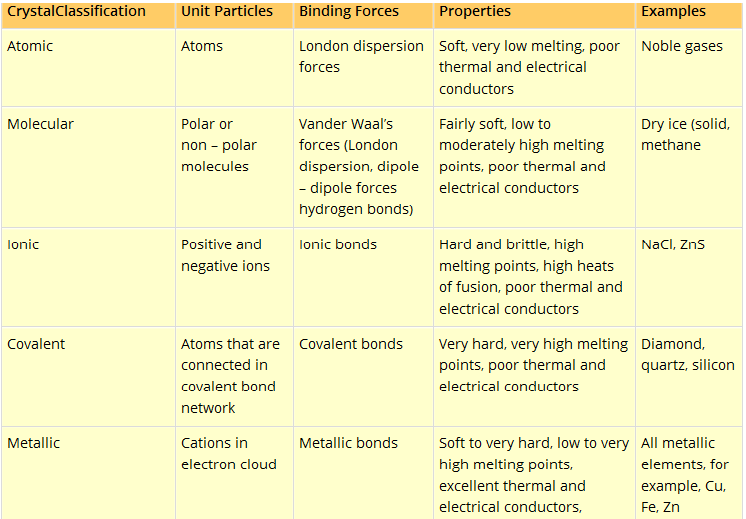
Details in a tabular form
Now let us classify our given solids and view their crystal structures.
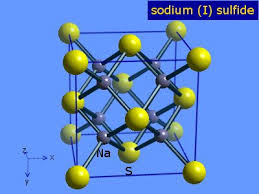
1.Sodium sulfide, #N_2S=>color(red)"(IONIC)"#
The constituent particles, #Na^+ and S^"2-"# ions are associated with ionic bondage .
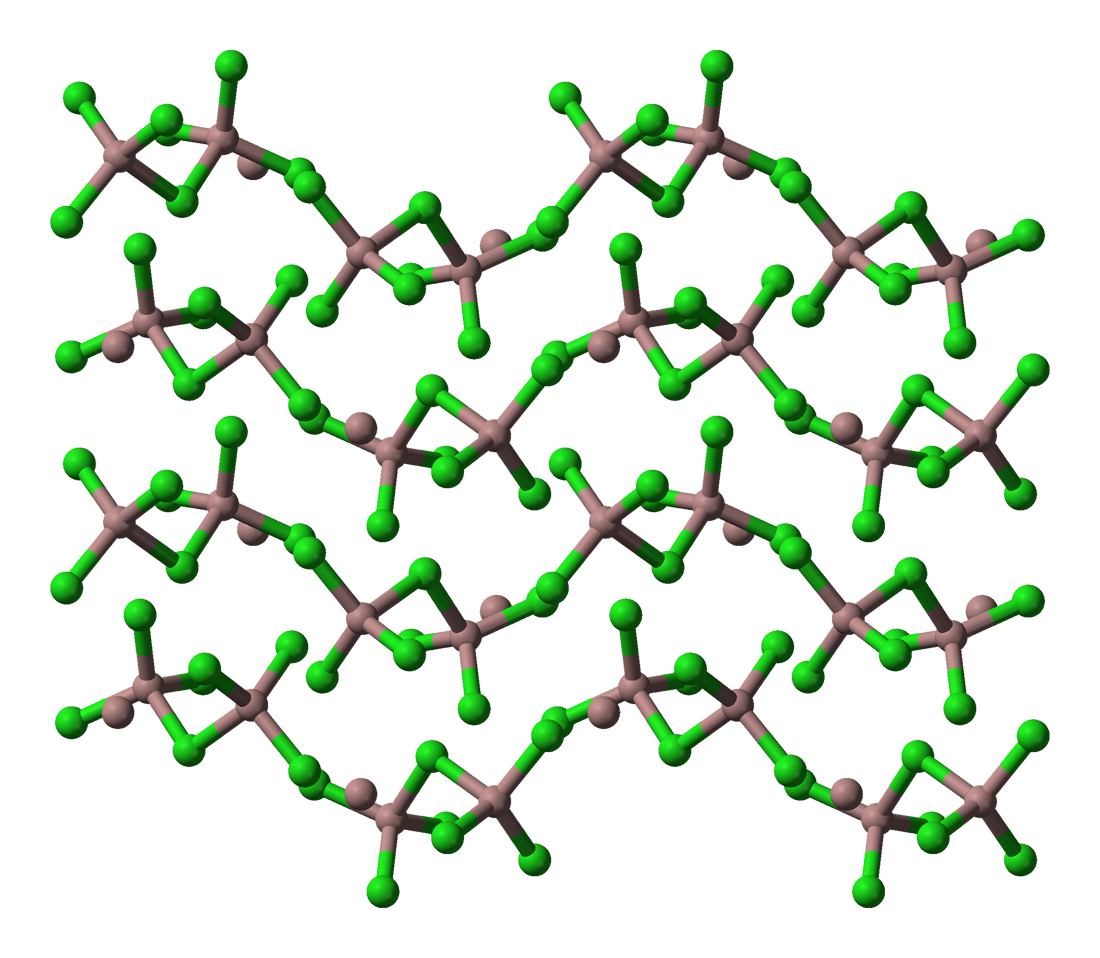
2. Carbon tetrachloride, #C Cl_4=>color(red)"(MOLECULAR)"#
The constituent particles are tetrahedral #C Cl_4# molecules associated with vander waal's forces .
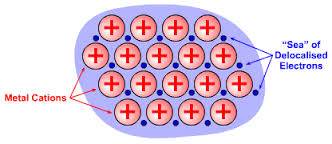
3, Calcium metal, #Ca=>color(red)"(METALLIC)"#
In this crystal #Ca^"2+"# ions are boded with metallic bonds , where cations are in the sea of delocalized valence electrons of the metal atoms existing in the piece of crystal.
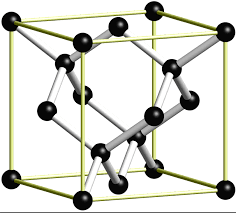
4. C(diamond), #=>color(red)"(COVALENT-NETWORK)"#
Here #C# atoms are connected incovalent bond network where each C atom is linked with 4 C atoms tetrahdrally by strong covalent bond .