What is the Lewis Dot Structure for #Mg_2^+#?
1 Answer
Feb 4, 2017
If I were to take you literally, it would be a rather unstable cation...
#["Mg"cdots"Mg"]^(+)#
(the dots are an interaction line for a half-bond, not electrons)
Its MO diagram would have one less valence electron than that for
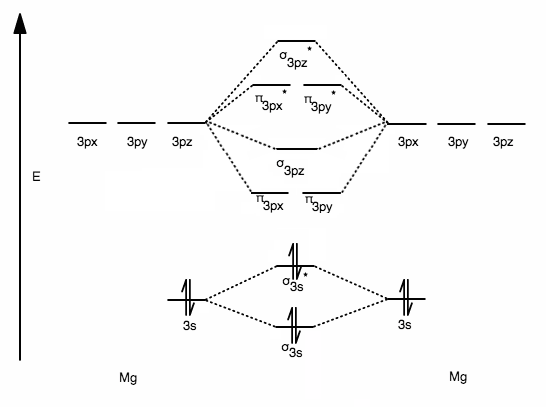
Take one valence electron out of the antibonding
Thus, MO theory suggests that
If you mean

