Which nuclide will decay by positron and what isotope will be the left over? Both answers must be correct. (Numbers represent the mass number): 32S, 32P; 26P, 26S; 14O, 14N; 14N, 14O
1 Answer
32P
26P
14O
This all things will decay due to positron emission
Explanation:
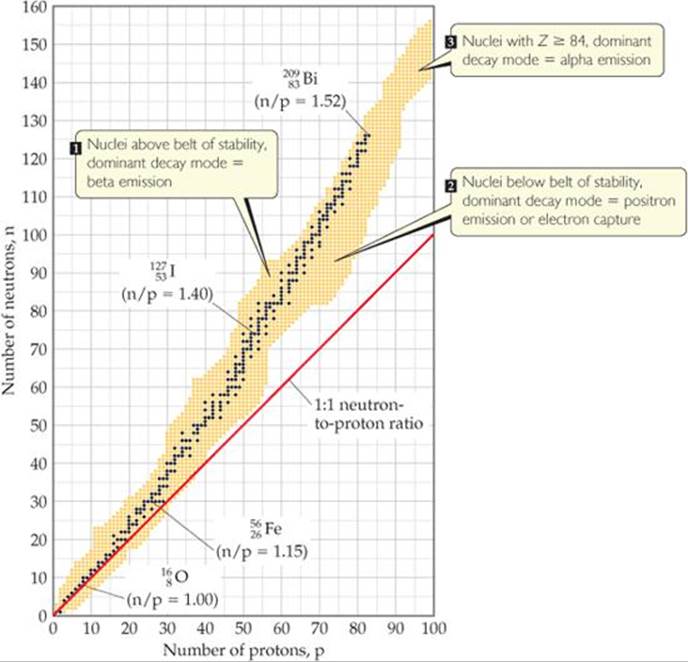
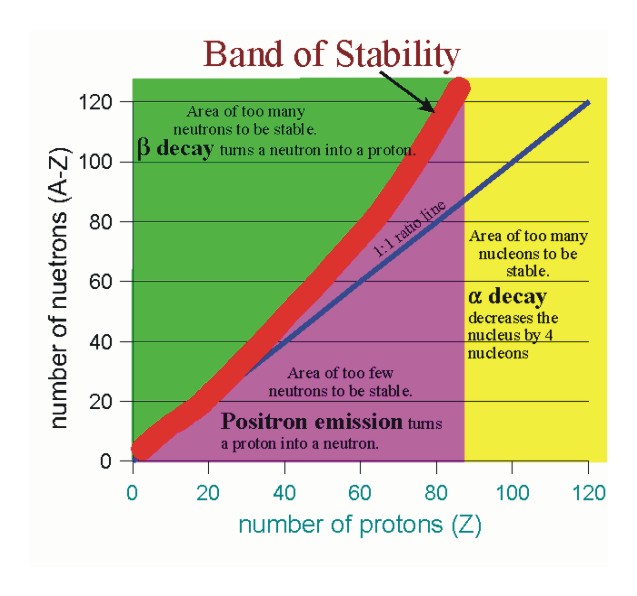
Laws of radioactivity
If
If
For atomic no. = 1
stable if
For atomic no.= 20
stable if
For atomic n= 40
stable if #N/Z ratio 1.5
For atomic no. > 83
no stable nuclei
32S has 16 neutrons , 16 protons
atomic no. = 16
so it will not decay
32P has 17neutrons and 15protons
atomic weight = 15
From this we know that 32P is not stable but type of radioactive decay is positron emission as 1.133< 1.5
26P
protons = 15
Z(protons) = 15
N(neutrons) = 11
26P is not stable but as 0.733 is too low than 1 it is a positron emission
26S No. of protons = 16
No. of neutrons = 10
Its not stable but still its a proton emission not positron emission.
But as 0.625<1 this radioactive decay follows a positron emission
14N
No. of protons = 7
No. of neutrons = 7
It is stable
14O
No. of protons = 8
No. of neutrons = 6
Too low than 1 so positron emission


