Question #29f82
1 Answer
See explanation.
Explanation:
The mass number,
In other words, the mass number tells you how many protons and neutrons are located inside the nucleus of a given atom.
#color(blue)(ul(color(black)("mass number = no. of protons + no. of neutrons")))#
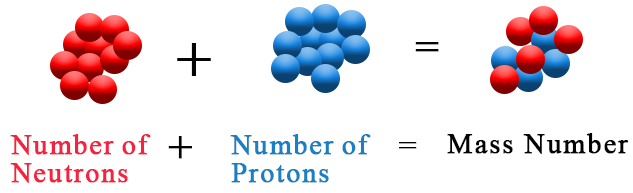
As you know, the number of protons present inside the nucleus is given by the atomic number,
You can thus rewrite the above equation as
#color(blue)(ul(color(black)(A = Z + "no. of neutrons")))#
The mass number of the most common isotope of an element can be calculated by using the value of the atomic mass, which is listed in the element's box in the Periodic Table of Elements.
Take, for example. silver,
As you can see, silver has an atomic mass of
In this case, you'd have
#107.87 ~~ 108 -># rounded to the nearest whole nubmer
This means that the most common isotope of silver is silver-108. This isotope has a mass number equal to
You know that silver has an atomic number equal to
#A = Z + "no. of neutrons" implies "no. of neutrons" = A - Z#
you can say that the silver-108 isotope has
#"no. of neutrons" = 108 - 47 = 61#
neutrons located inside its nucleus.


