Question #ff550
1 Answer
Explanation:
The idea here is that the atomic mass of an element is calculated by taking the weighted average of the atomic masses of its stable isotopes.
The atomic masses of these stable isotopes will contribute in proportion to their abundance.
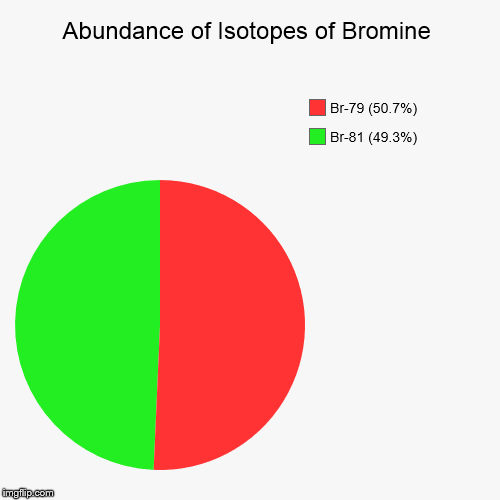
Since bromine is said to have
Remember, only stable isotopes are taken into account when calculating the atomic mass of an element, so it makes sense that their percent abundances must add up to give
#100% = "% abundance" color(white)(.)^79"Br" + "% abundance" color(white)(.)^81"Br"#
In your case, you know that
#"% abundance" color(white)(.)""^79"Br" = 50.69%#
which means that the percent abundance of
#"% abundance" color(white)(.)^81"Br" = 100% - 50.69% = color(darkgreen)(ul(color(black)(49.31%)))#