When dissolved in equal volumes of water, which of the solutions will have a higher pH?
If two equal numbers of moles of nitric acid and formic acid were dissolved in an equal volume of water, which one would have the higher pH?
If two equal numbers of moles of nitric acid and formic acid were dissolved in an equal volume of water, which one would have the higher pH?
1 Answer
The formic acid solution.
Explanation:
The idea here is that nitric acid,
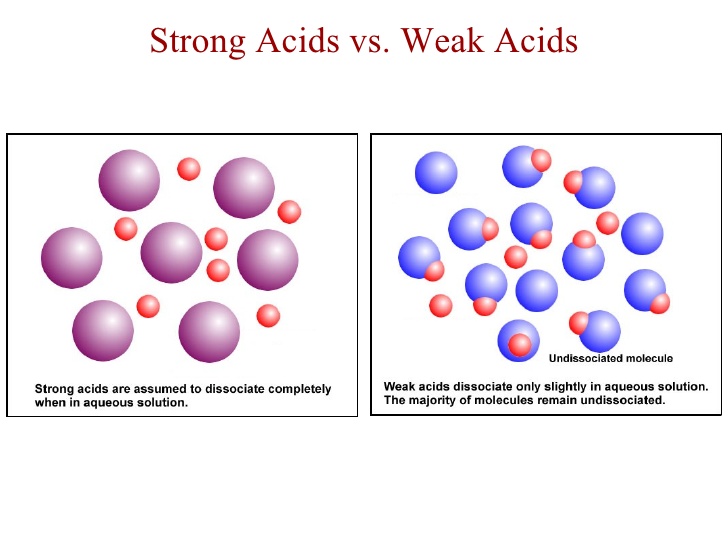
This implies that nitric acid dissociates completely in aqueous solution to produce hydronium cations,
#"HNO"_ (3(aq)) + "H"_ 2"O"_ ((l)) -> "H"_ 3"O"_ ((aq))^(+) + "NO"_ (3(aq))^(-)#
As you can see, every mole of nitric acid added to the solution will dissociate to produce
On the other hand, formic acid does not dissociate completely in aqueous solution to produce hydronium cations
#"HCOOH"_ ((aq)) + "H"_ 2"O"_ ((l)) rightleftharpoons "H"_ 3"O"_ ((aq))^(+) + "HCOO"_ ((aq))^(-)#
Formic acid has an acid dissociation constant,
#K_a = 1.77 * 10^(-4)#
This means that for the above equilibrium reaction, you have
#K_a = (["H"_3"O"^(+)] * ["HCOO"^(-)])/(["HCOOH"])#
Since
For equal numbers of moles of nitric acid and formic acid dissolved in equal volumes of water, the concentration of hydronium cations will be higher for nitric acid and lower for formic acid.
#["H"_ 3"O"^(+)]_ "nitric acid" > ["H"_ 3"O"^(+)]_ "formic acid"#
Since
#color(blue)(ul(color(black)("pH" = - log(["H"_3"O"^(+)]))))#
you can say that the higher the hydronium concentration in the resulting solution, the lower the pH. This implies that
#"pH"_ "formic acid" > "pH"_"nitric acid"#

