What is an acid chemical compound?
1 Answer
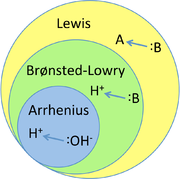
There are 3 main theories used to describe what an acid/ base is:
- Arrhenius theory
- Brønsted-Lowry theory
- Lewis Theory
Explanation:
Arrhenius theory describes an acid as a species that dissociates in water to produce an
Brønsted-Lowry theory describes an acid as a species that donates a proton (
Lewis theory describes acids as species that are able to accept electron pairs during reactions and bases as species that donate electron pairs during reactions.
These theories all work to some extent and some are more useful than others in certain circumstances. You can think of Brønsted-Lowry theory as expanding on Arrhenius theory because it includes all Arrhenius acids and bases plus a few more. Similarly Lewis theory expands on Brønsted-Lowry theory because it includes all Brønsted-Lowry acids and bases plus a few more.