Question #12162
1 Answer
Mar 3, 2017
There are 17 lone pairs in one molecule of
Explanation:
The structure of tetraphosphorus heptoxide,
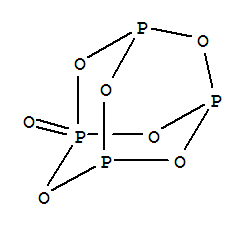 img1.guidechem.com
img1.guidechem.com
Each
Thus
There are 17 lone pairs in one molecule of
The structure of tetraphosphorus heptoxide,
 img1.guidechem.com
img1.guidechem.com
Each
Thus