Draw the Lewis electron dot structure for #SF_2#. How many lone pairs of electrons are on the central atom?
1 Answer
Mar 6, 2017
Explanation:
We have
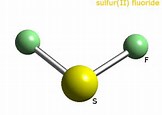
Given that the central sulfur has 4 electron pairs surrounding it, 2 bonding, and 2 non-bonding, VESPER predicts that these are arranged in a tetrahedron to a first approx. The
This site reports that

