What is the tendency of an atom to pull electrons toward itself referred to as?
1 Answer
Mar 11, 2017
You speak of the
Explanation:
The
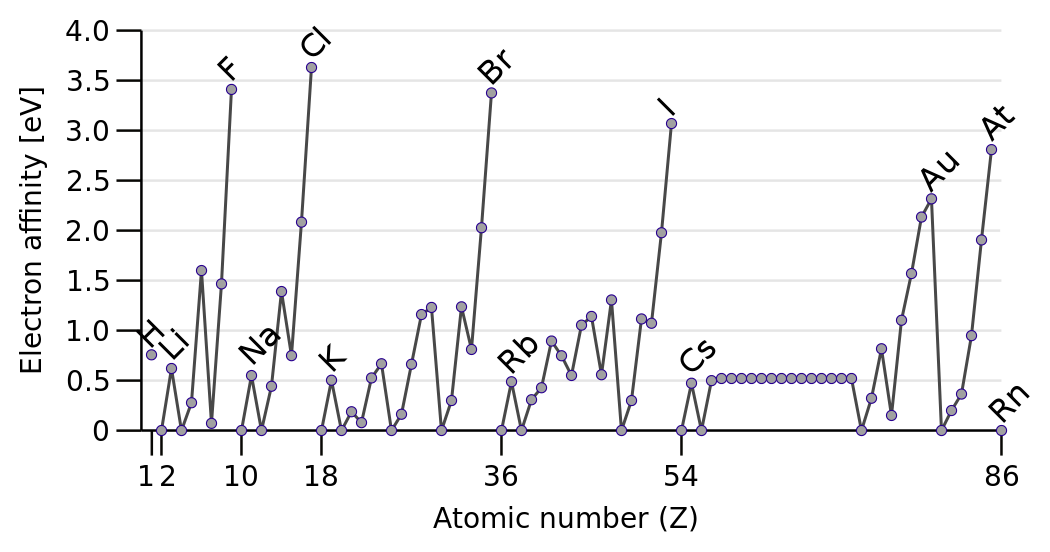
Discounting the Noble Gases, electron affinity INCREASES across the Period from left to right as we face the Table. Why should this be so?

