Given 100.0 g of a radioactive isotope that has a half-life of 25 years, what amount of that isotope will remain after 100 years?
1 Answer
Mar 30, 2017
After 100 years, 6.25g will remain.
Explanation:
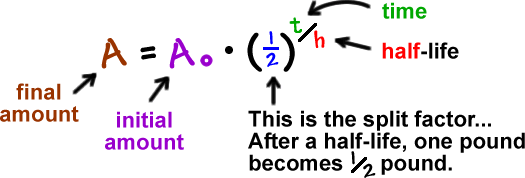
We can follow the half-life formula to solve.
Following the image, we have the formula
Now we'd plug in for each variable we have.
Our
Our
We currently have
We can plug all that into a calculator, or solve for the exponent first.
After 100 years, 6.25g will remain.