Question #1a111
1 Answer
Explanation:
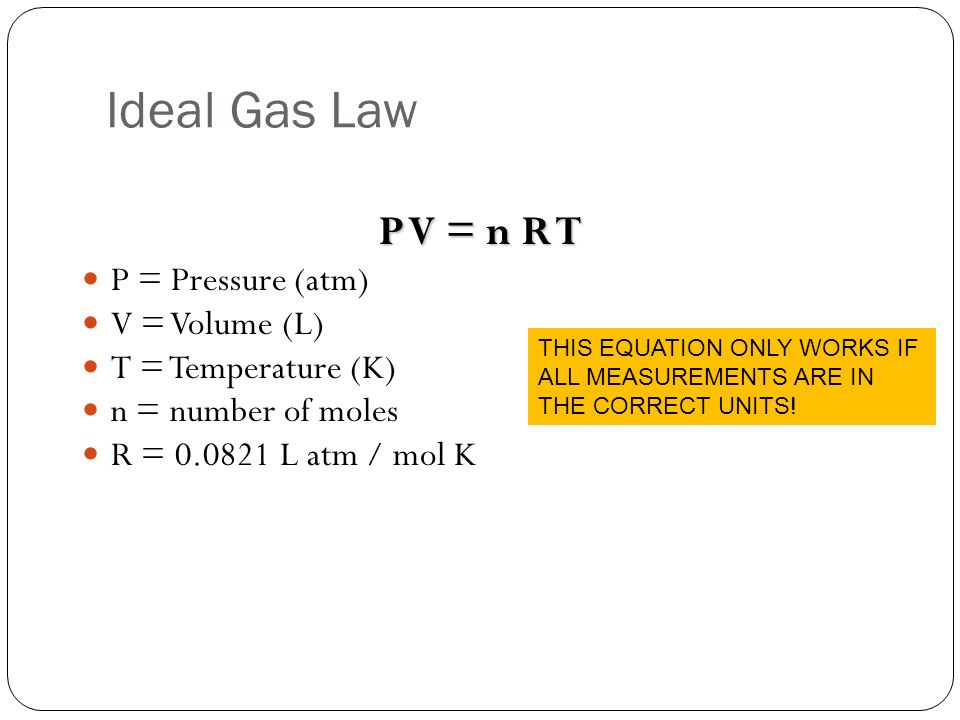
Because we are at standard temperature and pressure and are given only one set of conditions, we have to use the ideal gas law equation:
 slideplayer.com
slideplayer.com
I should mention that the pressure does not always have units of atm, it depends on the units of pressure given in the gas constant.
List your known and unknown variables.
- Number of moles
- Temperature
- Pressure
Volume of
At STP, the temperature is 273K and the pressure is 1 atm.
Because the ideal gas law deals with the number of moles instead of grams, we have to first convert grams of water vapor to moles of water vapor using the molar mass of water, which is 18.01 g/mol
Now, rearrange the equation to solve for V:

