Zirconium has an atomic number of 40. How many neutrons are there in an isotope of zirconium-92?
1 Answer
Mar 30, 2017
52
Explanation:
To determine the number of neutrons that zirconium-92 has we will use the formula below:
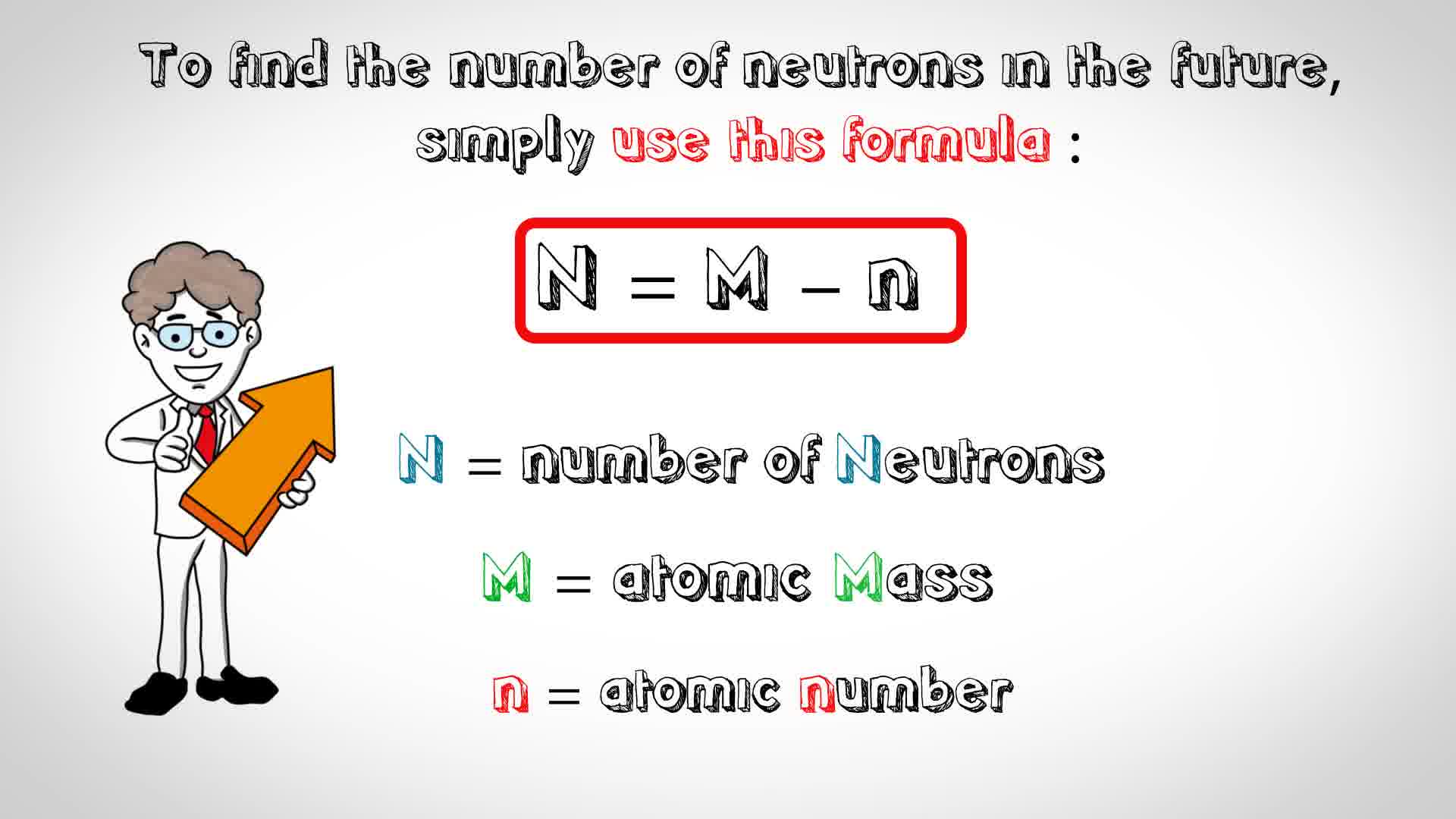 https://www.bing.com/images/search?view=detailV2&ccid=70Z1Wcya&id=D1BA60E19E8F3DA68872E6C69C41E9EE64709380&q=how+to+determine+number+of+neutrons&simid=608048868086907614&selectedIndex=2&ajaxhist=0
https://www.bing.com/images/search?view=detailV2&ccid=70Z1Wcya&id=D1BA60E19E8F3DA68872E6C69C41E9EE64709380&q=how+to+determine+number+of+neutrons&simid=608048868086907614&selectedIndex=2&ajaxhist=0
We know the atomic number (40) and we know the atomic mass (92), so all we have to do is plug the numbers into the equation and solve for N:
There are 52 neutrons in the isotope Zr-92
*I should also mention that the number to the right of the element's name is the atomic mass.
I hope this helps!

