How does the conductivity of the ionic and the covalent compounds compare?
1 Answer
Apr 1, 2017
ionic compounds are conductive in a water solution.
Explanation:
In a water solution of ionic compounds, the ions go into solution.
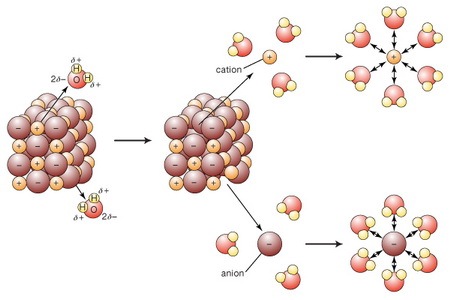
(From www.britannica.com)
These ions can act as conductors of electricity.

Water itself is a covalent molecule. Distilled water has a very low conductivity.
An aqueous solution of covalent molecules like sugar does not conduct any better than distilled water.

