What causes an unsaturated fatty acid to have a different shape than a saturated fatty acid?
1 Answer
Unsaturated fatty acids contain double bonded carbon atoms, which has a different bonding angle than the single bonded carbon atoms in a saturated fatty acid.
Explanation:
There are two types of fatty acids: saturated ones and unsaturated ones. Both are displayed below.
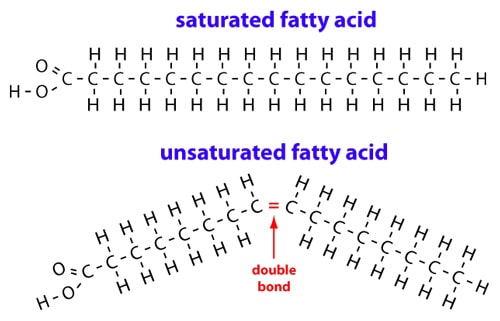
In the picture above, the saturated fatty acid is displayed in a straight line. The carbon atoms in the fatty acids have a certain bond angle, which is the same for every carbon atom in the
In the unsaturated fatty acid, there are one or multiple double bonds in the string on the right side of the
This change causes the fatty acids to have a different shape.
For your interest, an unsaturated fatty acid can contain multiple double bonds. These double bonds can undergo reactions which are for example crucial in the drying of biological paint (linseed oil).

