Is diamond an insulator? What about graphite?
1 Answer
Yes, this is true for pure diamond. Graphite contains double bonds that can move across the layer. These double bonds consist out of electrons with are needed to conduct electricity.
Explanation:
I hear you thinking, graphite and diamond both contain carbon atoms to each other, shouldn't they both conduct electricity?
And the answer is no, because of the structure these carbon atoms are different in diamond as in graphite. The answer to this question is quite complicated but definitely interesting!
Diamond
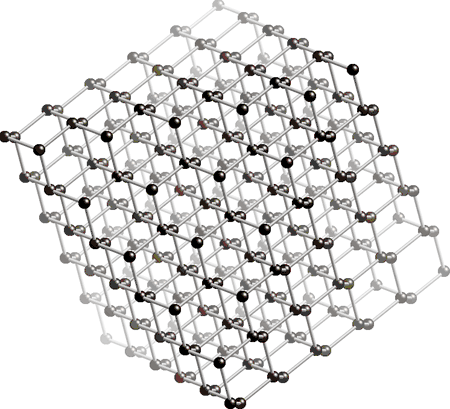
The structure of diamond is showed below. The black dots in the picture represents the carbon atoms. As you may be able to see, every carbon atom is connected to 4 other carbon atoms (in the middle, not on the sides). This is the reason why the diamond has such a high hardness. For now please remember that all carbon atoms have 4 single bonds and that there are no double bonds present.
Graphite
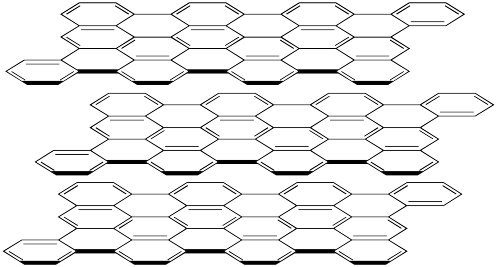
The structure of graphite is showed below. Here the carbon atoms are displayed on each side of a line, but not with a dot. (The bigger black lines only try to show us that the structure is drawn in 3D). We see in the image 3 different layers that are aligned on top of each other.
The carbon atoms of graphite form 4 bonds with 3 nearby carbon atoms. Therefore one of the bonds must be a double bond.
What do these double bonds have to do with the conductivity of the material?
The double bonds in the rings of graphite aren't set at a certain location on the layer. They can "move" across the molecule (Graphite has resonance structures, check out this page to learn more about resonance structures.).
Double bonds consist out of electrons, which in graphite can move through the layer.
Now image that you put an energy source over the graphite. At one side you putt electrons in the molecule and at the other side, you subtract the electrons from graphite. These electrons can move through the layer to the other side of the layer and back into the energy source creating a closed energy cycle! I tried to draw this process in the picture below to clarify.

And as you may have guessed: This process cannot happen inside (pure) diamond! No electron pushing trough the molecule and no electrical conductivity.
