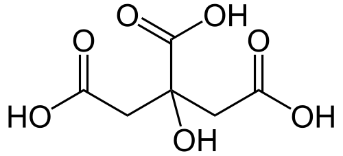
The structure of citric acid is
One of the first oxidation products is acetonedicarboxylic acid.
#underbrace("HOCOCH"_2"C(OH)(COOH)CH"_2"COOH")_color(red)("citric acid") → underbrace("HOCOCH"_2"COCH"_2"COOH")_color(red)("acetonedicarboxylic acid") + "CO"_2#
The equation for this reaction is
#underbrace("5C"_6"H"_8"O"_7)_color(red)("citric acid") + "2MnO"_4^"-" + "6H"^"+" → underbrace("5C"_5"H"_6"O"_5)_color(red)("acetonedicarboxylic acid") + "5CO"_2 + "2Mn"^"2+" + "8H"_2"O"#
Acetonedicarboxylic acid is readily decarboxylated to form acetone and carbon dioxide.
#underbrace("HOCOCH"_2"COCH"_2"COOH")_color(red)("acetonedicarboxylic acid") → underbrace("CH"_3"COCH"_3)_color(red)("acetone") + "2CO"_2#
Finally, acetone is oxidized to acetic acid and formic acid.
#underbrace("5CH"_3"COCH"_3)_color(red)("acetone") + "6MnO"_4^"-" + "18H"^"+" → underbrace("5CH"_3"COOH")_color(red)("acetic acid") + underbrace("5HCOOH")_color(red)("formic acid") + "6Mn"^"2+" + "9H"_2"O"#


