 google image modified
google image modified
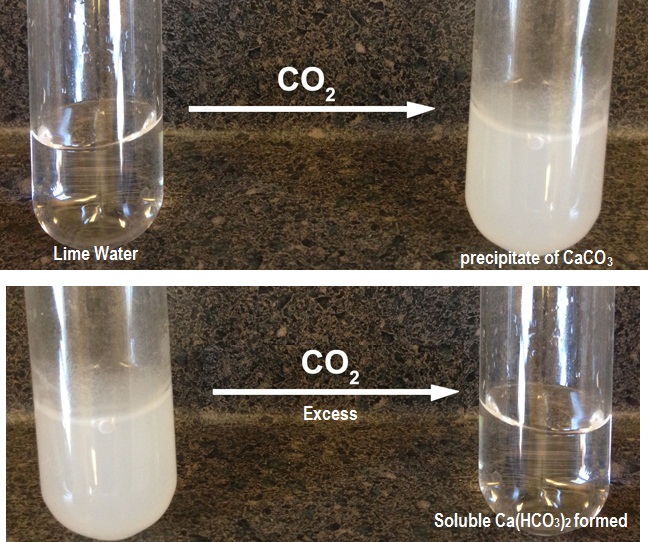
Saturated solution of lime has pH value 12.3. In this solution lime ionizes to form OH^- ions as follows
Ca(OH)_2(s)->Ca^(2+)(aq) + 2OH^(-)(aq)
When CO_2 (g) is passed through transparent lime water the acidic CO_2 (g) first tries to form H_2CO_3 with water. But H_2CO_3 formed readily interacts with OH^- in lime water and forms CO_3^(2-) in basic medium i.e at higher pH.
H_2CO_3+2OH^"-" ->CO_3^(2-)+2H_2O
This CO_3^(2-) ions thus formed combine with Ca^(2+) ions in solution and produce insoluble CaCO_3(s) as white precipitate and the mixture becomes turbid.
Ca^(2+)(aq)+CO_3^(2-)(aq)-> CaCO_3(s) darr
This salt establishes following heterogeneous equilibrium in solution
CaCO_3(s)rightleftharpoonsCa^(2+)(aq)+CO_3^(2-)(aq).....[1]
After precipitation of CaCO_3(s) is sufficient enough to lower the pH value of the solution considerably then the excess acidic CO_2 (g) passed through the solution interacts with CO_3^(2-)(aq) ions and water to form HCO_3^(-)(aq) as follows
CO_3^(2-)(aq)+H_2O+CO_2 (g)->2HCO_3^(-)(aq)
And thus due to removal of CO_3^(2-)(aq) from solution the equilibrium established before as per equation[1] is shifted towards right and dissolution of CaCO_3(s) occurs making the solution transparent again.
Reactions in two steps
Ca(OH)_2(s)->CaCO_3darr+H_2O
CaCO_3+H_2O+CO_2->Ca(HCO_3)_2
Please note
Here is how respected Ernest Z likes to modify the answer
Answer:
The excess carbon dioxide forms hydronium ion, which reacts with the carbonate ions from the calcium carbonate.
Explanation
When "CO"_2"(g)" is bubbled through water, it becomes involved in several equilibria:
"CO"_2"(g)" rightleftharpoons "CO"_2"(aq)"
"CO"_2"(aq)" + "H"_2"O(l)" rightleftharpoons "H"_2"CO"_3"(aq)"
"H"_2"CO"_3"(aq)" +"H"_2"O(l)" rightleftharpoons "HCO"_3^"-""(aq)" + "H"_3"O"^"+""(aq)"
"HCO"_3^"-""(aq)" +"H"_2"O(l)" rightleftharpoons "CO"_3^"2-""(aq)" + "H"_3"O"^"+"
A saturated solution of lime ionizes to form "OH"^"-" ions as follows
"Ca(OH)"_2"(s)" rightleftharpoons "Ca"^"2+""(aq)" + "2OH"^"-"(aq)
When "CO"_2"(g)" is passed through lime water, the "OH"^"-" reacts with the "H"_3"O"^"+"" and pulls the carbon dioxide equilibria to the right.
"CO"_2 + "2OH"^"-" -> "CO"_3^"2-" +"H"_2"O"
The "CO"_3^"2-" ions thus formed combine with "Ca"^"2+" ions in solution and produce insoluble "CaCO"_3"(s)" as a white precipitate, and the mixture becomes turbid.
"Ca"^"2+""(aq)"+"CO"_3^"2-""(aq)" -> "CaCO"_3"(s)"darr
 google image modified
google image modified
The calcium carbonate is involved in the following heterogeneous equilibrium:
"CaCO"_3"(s)"rightleftharpoons"Ca"^"2+""(aq)" + "CO"_3^"2-""(aq)"
As you continue to bubble in more "CO"_2, it forms more "H"_3"O"^"+".
The "H"_3"O"^"+" reacts with the "CO"_3^"2-" from the "CaCO"_3.
This pulls the "CaCO"_3 equilibrium to the right, and the "CaCO"_3 goes back into solution.
The overall reaction is
"CaCO"_3"(s)" rightleftharpoons "Ca"^"2+" + color(red)(cancel(color(black)("CO"_3^"2-")))
color(red)(cancel(color(black)("H"_3"O"^"+"))) + color(red)(cancel(color(black)("CO"_3^"2-"))) rightleftharpoons "HCO"_3^"-" + color(red)(cancel(color(black)("H"_2"O")))
"CO"_2 + color(red)(cancel(color(black)("H"_2"O"))) rightleftharpoons color(red)(cancel(color(black)("H"_2"CO"_3")))
color(red)(cancel(color(black)("H"_2"CO"_3))) + "H"_2"O" rightleftharpoons "HCO"_3^"-" + color(red)(cancel(color(black)("H"_3"O"^"+")))
stackrel(————————————————————)("CaCO"_3"(s)" + "CO"_2 + "H"_2"O" rightleftharpoons "Ca"^"2+" + "2HCO"_3^"-")
 google image modified
google image modified  google image modified
google image modified 
