How do I know if a double displacement reaction occurs?
2 Answers
- Two compounds with positive and negative ions.
- A difference in electro negativity or reactivity between the anions or cations.
- One of the new compounds in the product must be somewhat insoluble so that it comes out of solution.
Explanation:
- For a double replacement to take place there must be two positive and two negative ions.
-
A must be able to replace B. This will only happen if A is more reactive that B.
-
#(A^+Y^-) # must be insoluble so it forms a solid and comes out of solution.
You must make either (a) a precipitate or (b) a gas or (c) a nonelectrolyte.
Explanation:
A double displacement reaction is a chemical reaction in which two compounds react, and the positive ions (cations) switch partners with the negative ions (anions) to form two new products.
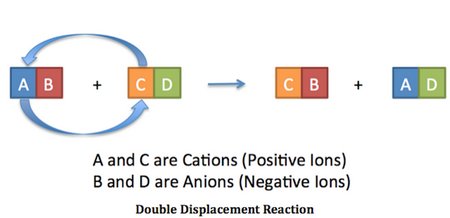 t
t
(From Study.com)
(a) Formation of a precipitate
(b) Formation of a gas
(c) Formation of a nonelectrolyte

