Question #5da9f
1 Answer
We use the normalised Boltzmann distribution in multiple dimensions to find the Maxwell-Boltzmann distribution.
Explanation:
The distribution of molecular speeds is based on the Boltzmann weight of translational motions and gives the probability of finding a particle with a certain velocity.
Under thermal equilibrium conditions, the probability
The normalised Boltzmann distribution is stated as following:
This function gives the probability of finding a particle with a certain velocity in a certain direction. Here I chose to find only the particles with velocity
Now we now that particles can move in three dimensions, not just in one. Therefore we need to find the distribution of speeds instead of the distribution of velocity.
Remember that the speed is the magnitude of the velocity vector.
The speed can, therefore, be calculated as:
Now using that the probability is proportional to the exponential of
Then we combine them and obtain the Maxwell-Boltzmann distribution of molecular speeds is therefore given by:
And as you can see in this equation we write
This equation gives the probability of finding a molecule with mass
If you plot this function you get
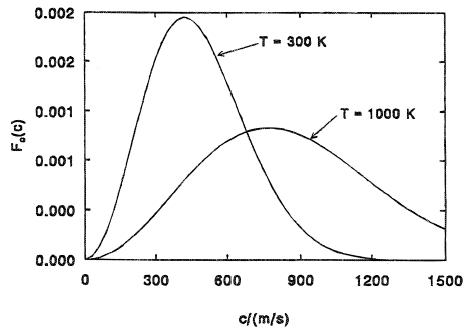
With the probability on the vertical axis and the speed of the particle on the horizontal axis.
