How do you determine if a pair of elements will most likely form an ionic compound?
1 Answer
Apr 11, 2017
A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. These types of ionic compounds are composed of monatomic cations and anions.
Explanation:
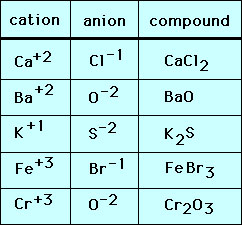
The chart below shows monatomic ions formed when an atom loses or gains one or more electrons, and the ionic compounds they form. You can check your periodic table to see that the cations are monatomic ions formed from metals, and the anions are monatomic ions formed from nonmetals.