Some drag-racing vehicles burn methanol, as presented by the following equation. What the mass of methanol that burns to produce an enthalpy change of #-9.00 xx 10^4# kJ?
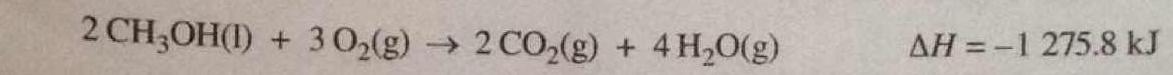
1 Answer
Approx.
Explanation:
Well, one way that is often useful conceptually is to regard energy,
EQUIVALENTLY..........
(I halved the equation to make the arithmetic easier! Of course I also had to halve the energy output given my approach.)
And we want an enthalpy change of
And thus the quotient,
And since for each mole of reaction, there is

