What kinds of titanium complexes can be made?
1 Answer
Apr 17, 2017
Some straightforward examples are
A cool example is the double decker titanium sandwich shown below (yum!):
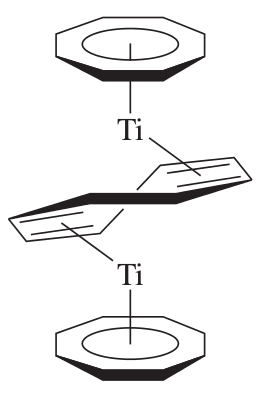
There are of course many others, but these are just a few.

