How does the ionization energy of an element evolve with respect to the Periodic Table?
2 Answers
Well in fact ionization energy INCREASES across a Period, from left to right as we face the Table.
Explanation:
For ionization energy, we measure the energy involved in the oxidation reaction:
And for a given Period, a given horizontal row on the Periodic Table, ionization energy INCREASES from left to right as we face the table.
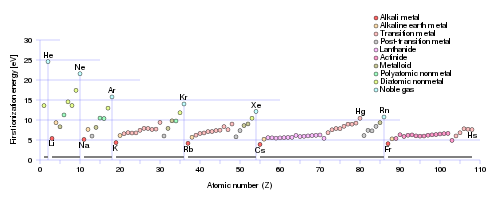
Incomplete electronic shells shield the nuclear charge VERY INEFFECTIVELY, and this is manifested by the clear increase in ionization energy ACROSS the Period. On the other hand, as we descend a Group, a column of the Periodic Table, ionization energy tends to decrease for the same electrostatic reasons. It is thus no surprise that the Noble Gases, with complete electronic shells, tend to exhibit the highest ionization energies.
Note that this same argument could be applied inversely to atomic SIZE, and for the same reasons. Atomic size decreases across the Period, and INCREASES down a Group.
Ionization energy increases with increasing atomic number.
Explanation:
-
On moving from LEFT TO RIGHT in periodic table :
-
The nuclear charge increases.
- The atomic size decreases.
-
The electrons will enter in same shell due to more nuclear charge.
-
Thus , ionization energy increases because energy to remove the electron from the shell will be greater because of great nuclear charge .
-
But , some irregularities in general trend have been noticed due to half filled and fully filled configurations having extra stability .


